Safety Assurance
Safety is a Top Priority
In order to produce cosmetic products that are wholeheartedly satisfied by our customers, at KOSÉ R & D Laboratories we conduct research and development from a variety of perspectives including; researching for new ingredients, formulation technologies, and new methods of usage. The most important matter for our laboratories is to ensure and create products with a high level of safety that are beneficial to our customer’s skin and health. Based on the belief of KOSÉ 's Behavioral Charter "Mind to Follow the Right Path" we have established strict and independent company standard to ensure the safety of our cosmetics.
Product Safety Assurance System
We offer quality products to our customers by conducting a safety assessment based on our strict internal safety standards. This assessment consists of two-tiered approach; the first evaluation for the safety of ingredients and then safety evaluation for actual products that use such materials. In the event that safety issues are reported in the market, we will respond to our customers through our storefronts and customer support service. Our Quality Assurance department will promptly share information appropriately within the company. We have built building an in-house mechanism so that such safety issues can be utilized to improve our existing products as well as in developing our new products.
Reference
Ingredients Policy (Sustainability)
Initiatives to assure quality in new product development – Assurance and safety as top priority – (Sustainability)
Our Policy on Assuring Safety of Cosmetics
The KOSÉ Group places product safety as its top priority and continues to promote the development of cosmetic products (including quasi-drugs) under our policy of not conducting animal testing.
*This excludes instances where we are held responsible by society to provide evidence for the safety of a product or where it is required by administrations in particular countries.
For details, please refer to this document.
Flow of Ensuring Cosmetic Safety
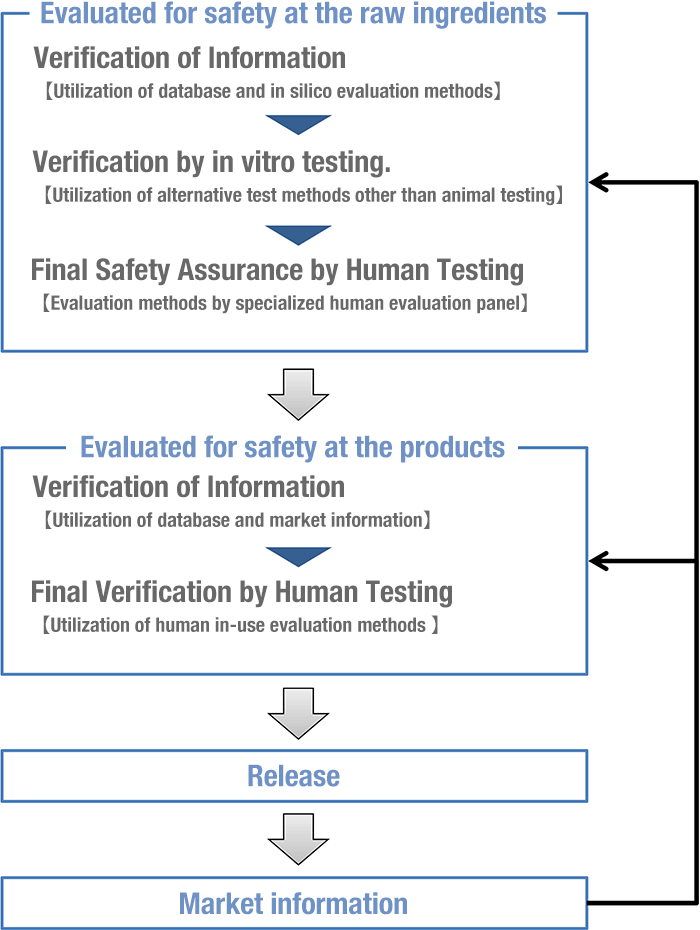
To ensure the safety of our cosmetics, we conduct a two-tiered assessment: one at the "ingredient" level and another at the "product" level.
All cosmetic ingredients are first evaluated for safety at the raw ingredient.
Based on the result of that first evaluation, we decide whether to use that ingredient or not. We develop our products using only the cosmetic ingredients that passed such evaluation. Furthermore, we always carry out a subsequent safety assessment at the product to comprehensively assess whether the product has any potential problem or has any issue with its usage etc. After completing this assessment then we decide whether or not this product can be released for sale.
Our safety evaluation methods are based on; confirmation of safety information collected from internal and external sources, confirmation by using alternative test methods such as in silico methodologies and/or in vitro testing, and confirmation by human testing.
We have built an in-house system so that our team can aggregate market information (customer's voice and inquiries from medical institutions) and reflect them on safety assessment of cosmetic ingredients and our products.
Steps for Ingredient Safety Evaluation
(1) Verification of Information [Utilization of database and in silico evaluation methods]
We will assess both internal and external information and investigate the safety risk of cosmetic ingredients. In addition to our accumulated knowledge which was acquired through past evaluations of ingredients and in-house information such as test data, we also make extensive use of external information such as thesis, reports by public organizations and databases.
Additionally, to predict toxicity, we conduct in silico simulation based on the chemical structure and properties of raw ingredients.
* in silico means "on computer".
(2) Verification by in vitro testing. [Utilization of alternative test methods other than animal testing]

We are advancing the development of cosmetics (including quasi- drugs) while placing safety as our top priority, adhering to a non-animal testing policy, and utilizing alternative test methods. In these alternative test methods, by using cultured cells and the like, we evaluate skin and eye irritation level and allergic reactions. For these tests, we utilize reconstructed human models or cells suitable for each test (e.g. a cultured human epidermal model or a cultured human corneal-epithelial model). We also use the similar assessment for the evaluation of product itself.
The safety evaluation process of alternative animal testing methods is still being developed all over the world. In Japan, KOSÉ along with various cosmetic manufacturers are also working on cooperation with alternative method development and its application.
-
Cultured cells used for an alternative test method -
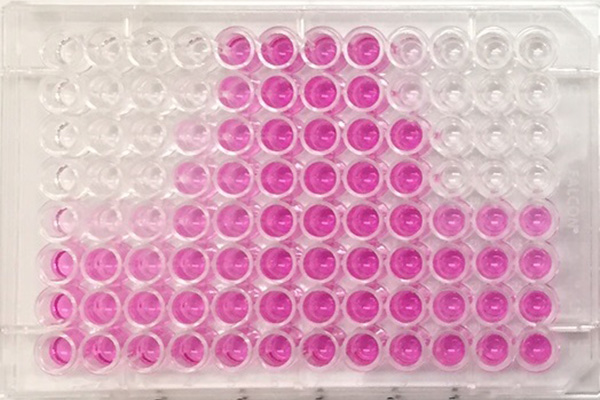
Measurement of cytotoxicity when evaluating skin irritation etc. (the darker the red pigment, the larger the number of live cells)
Main Safety Evaluation Method by in vitro Testing
- Eye irritation assessment, skin irritation assessment: Cell toxicity of cosmetic ingredients or products are measured. The potency of eye or/and skin irritation depending on the degree of cytotoxicity is evaluated.
- Phototoxicity assessment: The ingredients or products are irradiated with light (UV or visible light), then afterwards they are measured for phototoxicity to cells. The phototoxicity is evaluated by the degree of change in cytotoxicity when irradiated with light. Apart from this, there is also a method without using cells, which evaluates the photoreactivity by measuring reactive oxygen species generated when ultraviolet are irradiated to ingredients or products.
- Skin sensitization (Allergy) assessment: Cosmetic ingredients are evaluated for allergic properties in a test system that reproduces part of allergic reactions occurring in vivo. Due to the complexity of allergic reaction occurring in the whole body, several different test methods that correspond to each reaction are used in combination.
- Genotoxicity assessment: In this test, cosmetic ingredients are added to cells or microorganisms and the toxicity to DNA or chromosome is evaluated. Additionally, in this test, we use special microorganisms that cannot grow in normal circumstances. We then determine the genotoxicity by observing the number of bacteria that can survive by gene mutation, cellular chromosome count, and the degree of chromosomal structure changes.
Final Safety Assurance by Human Testing [Evaluation methods by specialized human evaluation panel]
After the verification in (1) and (2) above is completed, we conduct tests using a specialized human evaluation panel to confirm the ultimate safety to assure that there is no irritation or sensitization. Based on the results, we decide whether the cosmetic ingredients can be used in products.
The main human tests conducted during ingredient evaluation include; patch test, repeated insult patch test, stinging test (details of each test can be found here).
Steps for Product Safety Assurance
(1) Verification of Information [Utilization of database and market information]
When evaluating our products, we investigate safety risks from various information sources. In addition to the accumulated knowledge and test data from our own company, we also check market information such as sales performance of on-shelf products, inquiries from customers, as well as inquiries from medical institutions.
(2) Final Verification by Human Testing [Utilization of human in-use evaluation methods]
Similar to cosmetic ingredients evaluation, these tests are carried out by humans to assure product safety.
Particularly, during the product evaluation, in-use tests are conducted under conditions similar to normal use. Based on the results of such testing, we will then decide whether to release the product for sale and whether it will be accepted by our customers.
We also check whether various safety approval labels such as "(blank) tested" could be attached to the product.
Safety Assurance by Human Testing
〈Patch Test〉
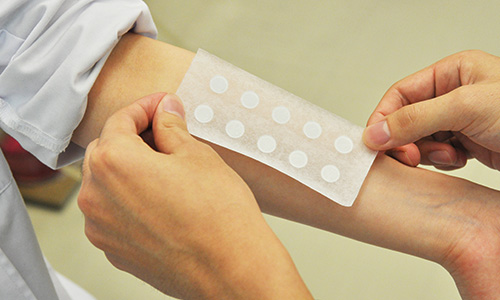

Patch test is conducted to evaluate the potential of an ingredient or product that may cause irritation to human skin. A small sample amount of ingredient or product being tested is placed on a special adhesive bandage, which is then patched on the skin of a subject for 24 hours or for 48 hours. The patch is removed and for up to next 5 days, a person who is experienced in reading patch test results will examine and scores the skin reactions such as redness or swelling under the patched area. In particular, for products marked as "Patch Tested", we perform this test and have a dermatologist make a judgment to confirm that the irritation is low. (This test does not guarantee that skin trouble will not occur to all people).
〈Repeated Insult Patch Test〉
Repeated insult patch test is conducted to evaluate allergenicity in human skin. To conduct this test, the subject must first undergo the patch test for three times a week for 3 weeks, and after a 2-week rest period, the subject must undergo another patch test again to confirm that no skin reactions occur. In particular, products that are marked as "Dermatologically Tested" must first clear a prescribed product standard which is more stringent than that of a regular product standard and then they must clear this test. (This test does not guarantee that allergies will not occur to all people).
〈Stinging Test〉
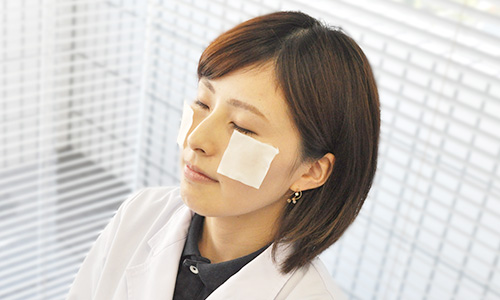
In cosmetics, "Stinging" refers to sensory stimuli such as tingling or itching and it is different from irritation associated with inflammatory symptoms such as rash or swelling. There are some people who feel such sensory stimulation with specific ingredients that are used in cosmetics.
Considering such people, we conduct the stinging test and verify that they do not feel sensory stimulation when using our products.
Stinging tests are performed on subjects who are sensitive to pre-selected sensory stimuli. A small sample of ingredients or products is applied to the subject's cheek or we ask the subjects to use the product in the manner as prescribed. The subject him/herself will declare the degree of sensory stimulation after a certain time.
In particular, for products that are marked as "Stinging Tested", we first ensure that they clear the appropriate prescription criteria and then verify that the degree of sensory stimulation is low (This test does not guarantee that skin trouble will not occur to all people).
〈Non-Comedogenicity Test〉
Comedo is a mixture of horns and lipid that looks like a white papule.
Comedones can clog the pore and they become the cause of acne in human skin.
Non-comedogenicity tests evaluate that comedones do not formulate against the human skin.
Testing is done either by repeating the patch test for 4 weeks or using the product under conditions similar to normal use and the tester evaluates whether comedones have formed on the skin. In particular, for products that are marked as "Non-comedogenicity testsed", we first ensure that they clear the proper prescription criteria and then follow up by the Non-comedogenicity tests to ensure that no comedones will form after using the product. (This test does not guarantee that comedones will not form on all people)
〈In-Use Testing〉

This is a test to verify that when a product is used under conditions of normal usage it does not cause any problem on the applied area nor the entire body.
After the subject used the product for a certain period of time, they will answer to questionnaires and other inquiries about their skin condition etc. If necessary, we may also measure various skin parameters such as water content of the stratum corneum.